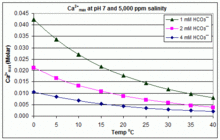
Solubility of calcium carbonate (lime scale) in water as a function of pH. | Download Scientific Diagram

Solubility of calcium carbonate (lime scale) in water as a function of pH. | Download Scientific Diagram
Thesis | The determination of the solubility of lime in water and of the sorption of lime on cellulose. | ID: v692t929f | eScholarship@McGill

Processes | Free Full-Text | Solubility Data of Potential Salts in the MgO-CaO-SO2-H2O-O2 System for Process Modeling
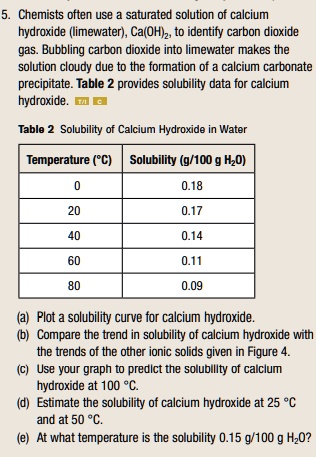
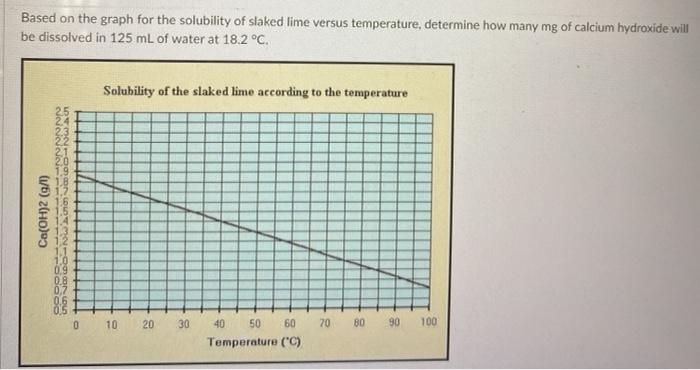
SOLVED: Chemists often use a saturated solution of calcium hydroxide (limewater), Ca(OH)2, to identify carbon dioxide gas. Bubbling carbon dioxide into limewater makes the solution cloudy due to the formation of calcium
The Solubility of Lime in Aqueous Solutions of Sugar and Glycerol | The Journal of Physical Chemistry
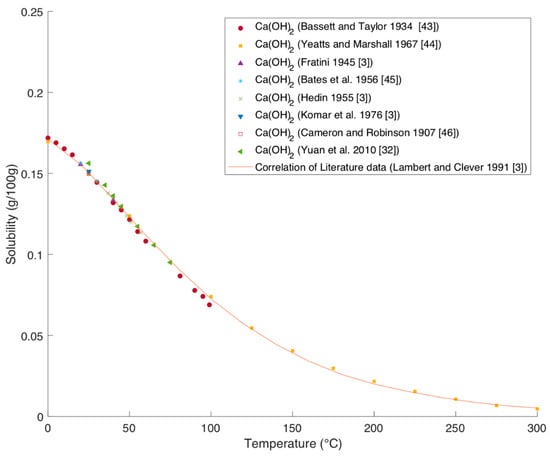
Processes | Free Full-Text | Solubility Data of Potential Salts in the MgO-CaO-SO2-H2O-O2 System for Process Modeling
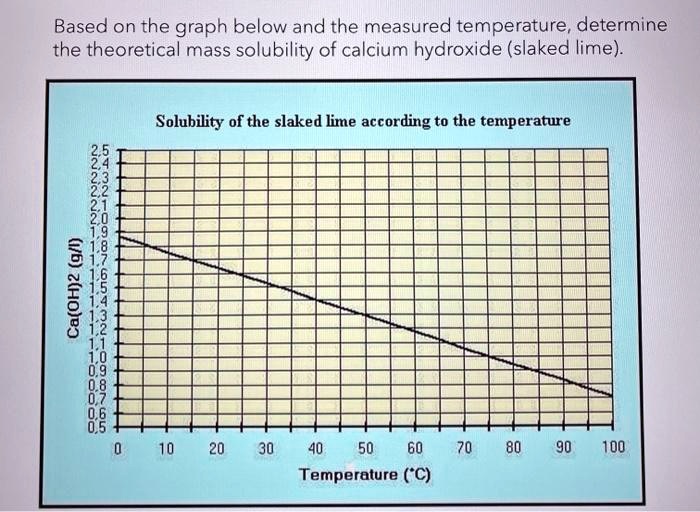
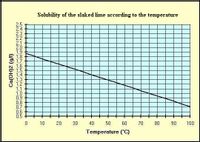
SOLVED: Based on the graph below and the measured temperature, determine the theoretical mass solubility of calcium hydroxide (slaked lime). Solubility of the slaked lime according to the temperature: 2H925612 0.9 0.8


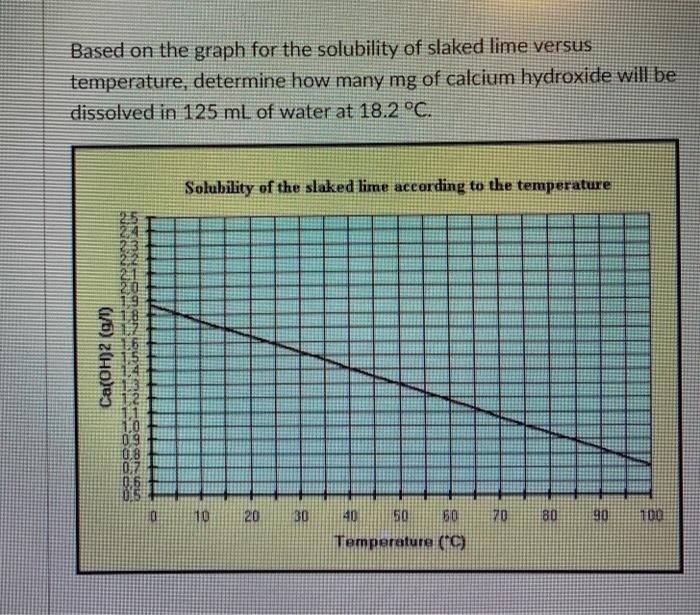
![Molar solubility of Ca (OH)2 in a solution that has a pH of 12. [ KSP [Ca (OH)2 ] = 5.6 × 10^- 12 ] Molar solubility of Ca (OH)2 in a solution that has a pH of 12. [ KSP [Ca (OH)2 ] = 5.6 × 10^- 12 ]](https://dwes9vv9u0550.cloudfront.net/images/10252669/718b1df9-54ff-4873-a48b-056b8accddcf.jpg)